Highlights
- Prior to beginning hormone therapy it is recommended that patients have an official diagnosis of gender dysphoria made by a mental health professional.
- The mainstay of cross-sex hormone therapy in FtM patients is testosterone (either topical or injectable).
- Absolute contraindications for hormone therapy must be ruled-out prior to initiation of treatment.
- Ever effort should be made to control medical conditions that may be exacerbated by testosterone prior to the initiation of testosterone.
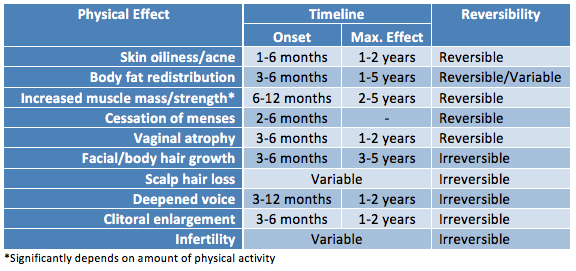
- The physical changes caused by testosterone vary from patient to patient and range from irreversible to reversible. The total physical transition takes approximately two years.
- Routine follow-up is required with regular bloodwork and testosterone dose adjustments as needed.
Overview
- Assessment
- Hormone Contraindications
- Hormone Risk Management
- Fertility
- Hormone Effects
- Hormone Regimens
- Patient Readiness
- Monitoring and Follow-up
Assessment
The assessment period is important for establishing a rapport with the patient, and establishing goals of treatment. The assessment period usually takes place over several office visits although the number of visits or time in between visits is less important than ensuring all areas are covered.
Fast Tracking:
It may be appropriate to shorten the assessment period in the following circumstances:
- A patient who is already using illicit hormones;
- A patient who has marked distress regarding their gender presentation;
- A patient whose medical and/or gender history is well known to you prior to them seeking hormone therapy;
- If another knowledgeable provider has recently performed part or all of the assessment and the associated records are available for review or a conversation with the prior provider can be arranged.
Before beginning a discussion surrounding the details of hormone therapy it is important to have addressed the following areas:
- History and physical exam
- Mental health: Ensure all mental health concerns have been adequately addressed by yourself or another qualified mental health professional; an official diagnosis of gender dysphoria is also most commonly attained prior to beginning treatment and is a requirement prior to a specialist referral in Moncton.
- Screening and Preventative care: Ensure all aspects of the patient’s health have been reviewed and addressed as needed. It is especially important to minimize the risks of hormones by maximizing overall health (smoking cessation, regular exercise, weight loss, etc.). In addition, an ECG should be completed in patients over age 40 and a stress test should be considered if the patient has risk factors for coronary artery disease.
- Baseline bloodwork: CBC, liver transaminases, creatinine, urea, electrolytes, glucose, cholesterol, testosterone, estradiol, prolactin, luteinizing hormone, Hepatitis B, C screening, pregnancy test.
- There is no consensus as to what gender should be listed on a laboratory requisition. For a patient who has undergone neither medical nor surgical management, the natal sex is usually used. If a patient has undergone medical or surgical interventions, the use of the sex opposite to their natal sex is recommended (i.e., M for FtMs and F for MtFs). For patients who are currently transitioning or have partially transitioned, one may need to vary the reported sex depending on the lab test. For example, for MtF patients beginning feminizing therapy, male laboratory norms may be more appropriate for creatinine and cholesterol, but female values may be more relevant for testosterone levels.
Hormone Contraindications
After a history, physical, and bloodwork have been completed you should have all the information you need to determine if the patient has any contraindications to hormonal therapy.
Absolute contraindications to testosterone:
-
- Pregnancy (non-pregnant patients require highly effective birth control; see “Fertility” below)
- Breastfeeding
- Unstable coronary artery disease
- Active endometrial cancer (exogenous testosterone may either increase or decrease the endometrial lining)
- Active known androgen-sensitive cancer
- Hypersensitivity to one of the formulation components
- Poorly controlled psychosis or acute homicidality
- Psychiatric conditions that limit ability to provide informed consent
Hormone Risk Management
A number of health risks have been thought to be related to testosterone therapy and some pre-exisiting medical conditions and risk factors may increase the risks associated with testosterone. The level of risk depends on the medication itself (dose, route), and the patient’s clinical characteristics (co-morbidities, family history, health habits). It is important to discuss risks and benefits with the patient and take reasonable measures to reduce all risk factors associated with relevant conditions. While ideally it is best to delay hormone treatment until major risk factors are controlled, denying access to hormones can do much more harm than some of the possible risks of treatment. With informed consent and appropriate support many providers will initiate hormones in patients with higher risk profiles when the improved quality of life/chance of survival outweighs the potential risks of hormones.
Risks:
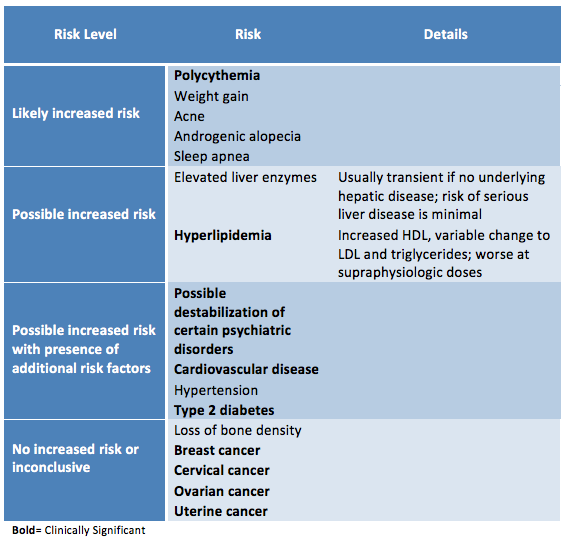 |
| Adapted from the WPATH Standards of Care version 7 |
Management:
- Stable ischemic heart disease: Consider cardiology consult, ensure optimal medical and/or surgical management as indicated, optimize risk factors, consider transdermal and/or lower dose testosterone.
- Uncontrolled hypertension: Address barriers to control and start antihypertensives as needed, consider a cardiac stress test. Encourage deferral of testosterone initiation until adequate control achieved (BP < 140/90).
- Uncontrolled diabetes: Address barriers to control, refer to a dietician, encourage lifestyle changes, start antihyperglycemic agents as indicated, consider cardiac stress test. Encourage deferral of testosterone initiation until adequate control achieved.
- Uncontrolled hyperlipidemia: Address barriers to control, refer to a dietician, start antilipemic agents as indicated, consider cardiac stress test, consider endocrinology referral, consider prophylactic aspirin in patients at high risk for developing cardiovascular disease. Encourage deferral of testosterone initiation until adequate control achieved.
- Framingham calculations are less reliable in the setting of exogenous hormones, but it is reasonable to consider using high-risk category lipid targets in transmen who have any risk factors for cardiac disease.
- Hepatic dysfunction: Address etiology as indicated (decreased alcohol consumption, weight loss if non-alcoholic fatty liver disease), consider GI/hepatology referral.
- Polycythemia: Identify etiology and address risk factors, consider low dose ASA, refer to hematology, consider transdermal testosterone. Encourage deferral of testosterone initiation until adequate control achieved.
- History of venous thromboembolism, hypercoagulable state: Minimize risk factors, monitor hemoglobin and hematocrit closely, consider transdermal testosterone.
- Chronic respiratory disease that may be worsened by erythrocytosis: Consider respirology referral, monitor hemoglobin and hematocrit closely, consider transdermal testosterone.
- Severe/uncontrolled sleep apnea: Start CPAP or other device, encourage weight loss if overweight, consider uvulopalatoplasty, monitor changes in CPAP pressure requirements.
- Androgen-sensitive epilepsy: Refer to neurology.
- Smoker: Encourage smoking cessation and offer medical assistance or negotiate a decrease in smoking, consider cardiac stress test (especially if additional cardiac risk factors are present), consider transdermal testosterone.
- Migraines: Consider neurology referral, consider daily migraine prophylaxis, consider transdermal testosterone.
- Inter-menstrual bleeding: Consider pelvic ultrasound (transvaginal if possible), consider gynecology referral (especially if risk factors for endometrial cancer).
- Oligo-/Amenorrhea: Consider pelvic ultrasound (transvaginal if possible), consider progesterone-induced menstrual bleed prior to testosterone initiation to thin endometrial lining.
- Obesity: Encourage weight loss, consider referral to a dietician, encourage regular exercise.
- Psychiatric disorders (testosterone may cause destabilization): Psychiatric comorbidities should be managed prior to the start of hormone therapy. However, there may be cases where a patient who is at a significant risk of suicide may in fact benefit greatly from the initiation of hormone therapy. This may be particularly true when intense gender dysphoria is the main source of the psychological distress experienced by the patient. Ensure appropriate mental health support is available to these patients and refer to psychiatry if necessary.
- Substance abuse: This puts patients at increased risk of complications. Stopping the substance completely is not always possible prior to the start of hormone therapy, therefore a harm reduction approach may be necessary.
- Estrogen-sensitive neoplasms: Testosterone is converted to estrogen via aromatization and may increase risk in patients with history of estrogen-dependent cancer. Consider oncologist referral.
- Polycystic ovarian syndrome: Many of it’s associated conditions can be exacerbated by testosterone and should be addressed prior to starting hormone therapy.
Fertility
Another important risk to address is how hormone treatment may effect reproduction. Patients who never underwent puberty of their natal sex because they took hormone blockers (GnRH analogues) did not develop the secondary sex characteristics to produce the gametes of their natal sex. However, in patients who did undergo puberty of their natal sex, if that individual has not had complete gender-affirming surgery, it may be possible to stop hormones long enough for natal hormones to recover, allowing the production of mature gametes. It is unknown how long a patient would need to be off hormone therapy for this to potentially occur. Success in releasing an egg after briefly stopping testosterone therapy depends on the patient’s age and duration of treatment. Options should be discussed with the patient prior to initiating hormone therapy. Patients should be informed about oocyte or embryo freezing which could later be used with a surrogate to carry the pregnancy. While there is availability of these services in Moncton, the cost is not covered.
Birth Control
In FtM patients, testosterone therapy does not prevent pregnancy even if amenorrhea is achieved, and testosterone is a teratogen. Reliable contraception may be required depending on sexual practices. Appropriate options include progesterone only contraception or an intrauterine device (IUD). It may be easier to insert an IUD prior to initiating testosterone, due to the subsequent atrophic changes of the vaginal and cervical tissues.
Hormone Effects
Hormone therapy will lead to many physical changes on a variable timeline that lasts on average two years. Physiologic responses to hormone therapy are quite variable and it is impossible to predict exactly what changes each patient can expect. The underlying body structure will not change with hormones. Trans men will maintain existing body and facial bone structure and hormonal treatment of adults does not impact a person’s height. Changes that can occur include: deepened voice, clitoral enlargement, growth in facial and body hair, cessation of menses, atrophy of breast tissue, increased libido, and decreased percentage of body fat compared to muscle mass. Changes range from reversible to irreversible. It is important that patients have realistic expectations regarding the possible changes with hormones.
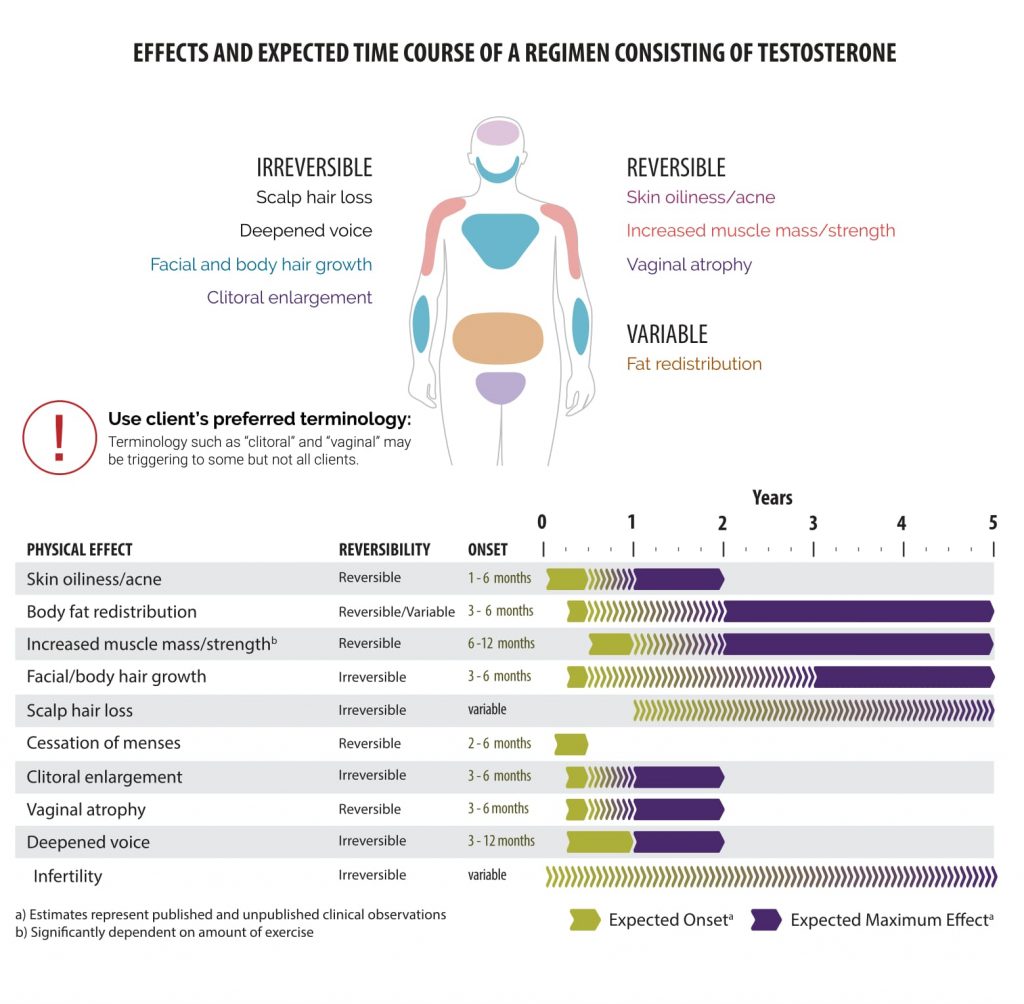 |
| Courtesy of Rainbow Health Ontario |
Sexuality
Exogenous hormones may have an impact on sexual attraction. Patients may notice an expansion of their sexual interests or a shift in their sexual orientation, which can be transient or permanent. This is often unforeseen and may create challenges for existing relationships. This effect has been most commonly noted with masculinizing therapies, but should be discussed as a possibility in all patients considering hormone therapy. Integrating an ongoing sexual health history is helpful in assessing for changes in risks for sexually transmitted infections and unplanned pregnancy.
Hormone Regimens
No controlled clinical trials have been conducted to assess the safety or efficacy of feminizing/masculinizing hormones in producing physical transition. A wide variety in dosing and types of hormones exists. Selecting the appropriate hormonal therapy will depend on patient preferences, patient goals of therapy, pre-existing medical conditions, and cost. Expected rate and degree of physical change will depend on dose and route of administration, age, genetics, body habitus, and lifestyle.
The main medication used in FtM transition is testosterone. It is available as an injectable or a transdermal preparation (patch or gel), but the injectable formulation is more common. Injectable testosterone is suspended in oil, which makes the buttocks the preferred site of injection, however this makes self-administration problematic. If patients are willing to inject half the volume weekly, then most patients are able to self-inject in the thigh (vastus) muscle.
Patients using topical gel preparations should be counselled regarding the risk of inadvertent exposure to others who come into contact with the patient’s skin, especially children and/or women who are pregnant or considering pregnancy. This risk can be reduced by applying the gel to areas that will be covered by clothing, allowing the gel to dry prior to getting dressed, and thorough hand washing following application.
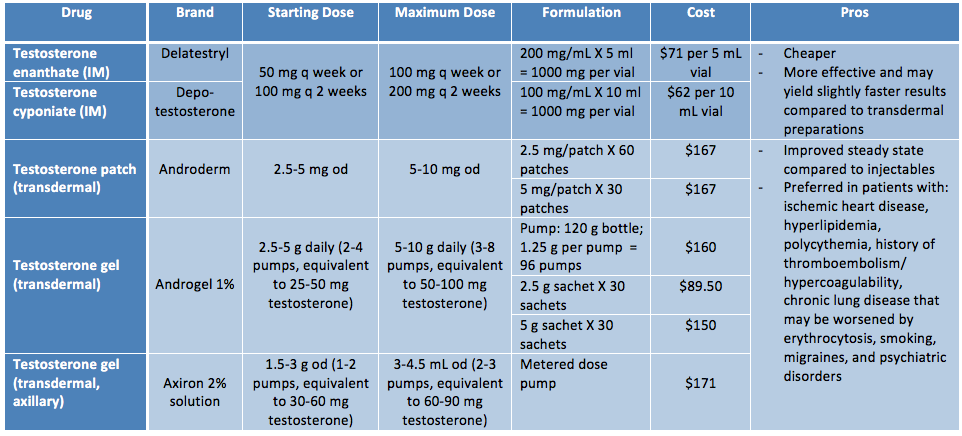 |
| Adapted from Rainbow Health Ontario |
Patient Readiness
Ensure that the patient has realistic expectations about what type of changes are possible as well as the timeline they can expect the changes to occur. Ensure the patient has a thorough understanding of the possible risks and complications of hormone therapy and that they understand that in some circumstances, they may develop a medical condition in the future that precludes them from continuing hormone therapy (e.g., certain types of cancer). It is also important to discuss fertility with all patients prior to starting hormonal therapy.
Gender transition and hormonal therapy represent significant change in a patient’s life and psychosocial support is important. Although not required for hormonal therapy, many patients may benefit from a trans-positive therapist.
Patients may choose to undergo a social transition either before, after, or in parallel with cross-sex hormone therapy. Social transitioning is a period of time in which transgender individuals live full-time in their affirmed gender. The purpose of this is to help patients confirm their affirmed gender and evaluate their ability to function as a member of that gender. It also provides insight into the adequacy of their social, economic, and psychological support. During social transitioning, the person’s feelings about the social transformation (including coping with the reaction of others) is a major focus of the counselling.
Additionally, patients should be encouraged to discuss their transition with family and friends as they too will be impacted by the patient’s transition. Consider asking the following questions:
- Who makes up your support system? How readily accessible are they?
- Do you know anyone else who has transitioned? What were the major struggles they had?
- What are the challenges you foresee with your family/friends?
- How will you manage your transition at work/school?
- How can you address those issues?
Attaining Consent
A patient must demonstrate an understanding of the risks and benefits of hormone therapy as well as existing alternatives. They must understand that physical transition is variable and no guarantees about final appearance can be made. Consent forms are not used by all physicians in Moncton but they are recommended by the WPATH. A sample consent from for male hormones is available here, adapted from the template by the Sherbourne Health Centre.
Monitoring and Follow-up
See Appendix F and G in the Guidelines and Protocols for Hormone Therapy and Primary Health Care for Trans Clients for a preventative care checklist for trans men.
Patients should be followed up at 1, 3, 6, and 12 months during the first year and then every 6-12 months thereafter, or more frequently if needed. Each follow-up should consist of a functional inquiry, targeted physical exam, preventative care/counselling, and bloodwork. The best assessment of efficacy of the hormone therapy is the clinical response – the patient’s physical changes. However, in order to more rapidly predict the appropriate hormone dosages the hormone levels are often used as guidance.
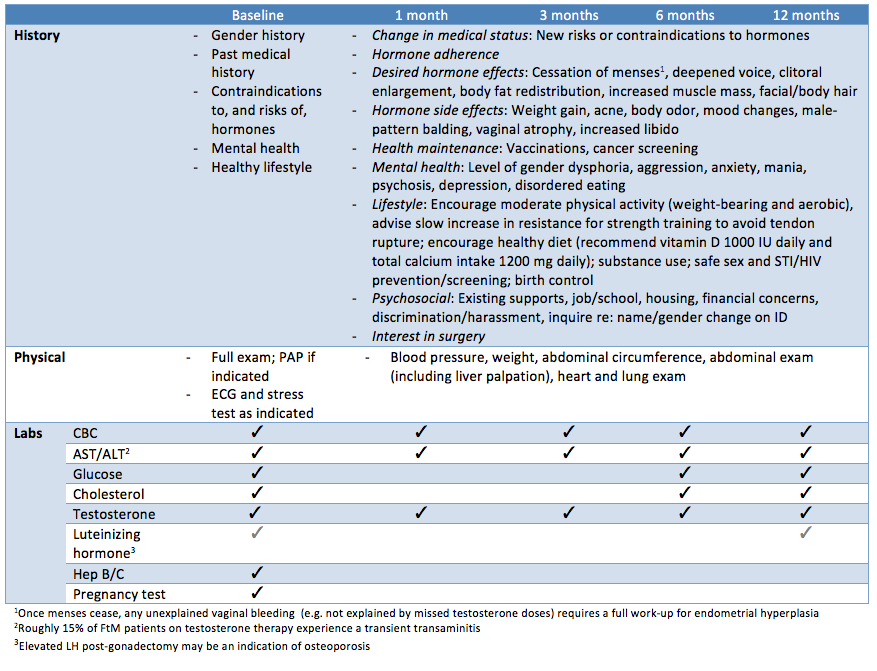 |
| Adapted from Rainbow Health Ontario |
Testosterone titration usually occurs in the early phases of treatment (1 and 3 months). A sample titration schedule would be: 25 mg testosterone x 4-6 weeks, then 50 mg testosterone x 4-6 weeks, then 75 mg testosterone, etc.) Some clinicians check injectable testosterone levels during the trough (just before the next injection) while others prefer midcycle. Whichever option is chosen, be sure it is consistent and that the patient understands exactly when bloodwork should be done. For transdermal testosterone, the testosterone level can be measured no sooner than after one week of daily application (at least two hours after application).The goal is to increase the testosterone into the physiologic male range (400–700 ng/dL). However the clinical effects are more important than the specific lab values. If the patient is happy on a subphysiologic dose of testosterone there is no need to increase the dose. Supraphysiologic levels should be avoided due to the increased risk of: erythrocytosis, sleep apnea, hypertension, excessive weight gain, salt retention, lipid changes, and excessive or cystic acne. At supraphysiologic levels, there is also the potential for the aromatization of excess testosterone into estrogen.
Once patients have achieved maximal masculinizing benefit from hormones (typically two or more years) they remain on a maintenance dose. Maintaining body changes generally requires lower hormone doses compared to initial induction. The maintenance dose is then adjusted for change in health conditions, aging, or other considerations (e.g., lifestyle changes).
Patients using topical preparations may require ongoing dose increases as the skin changes in order to obtain/maintain physiologic changes. If patients are needing a large volume of gel, compounding pharmacies can create a more concentrated formula (eg. 5% as opposed to 1%). Axillary gel could also be considered in this circumstance.
Reduction of testosterone needs to be considered if bloodwork reveals hepatic dysfunction or polycythemia. If polycythemia occurs in a client using injectable testosterone, increasing the dosing frequency to minimize the peak/trough may resolve the problem (half-life of testosterone is 8-9 days). An example would be to switch to 100 mg ever week rather than 200 mg every two weeks. Another option would be to switch to the transdermal route. Clinically significant hypertension or hyperlipidemia can be managed as in cis males without a testosterone dose reduction.
Acne is generally worse in the first few years of treatment and can be managed as in cis males. Severe acne may be improved by changing the formulation, route, and/or frequency of testosterone. Dose reduction only needs to be considered when all other treatment options have been exhausted.
Androgenic alopecia can be a result of testosterone therapy but is generally genetically determined. Thinning is also related to the duration of therapy. Finasteride may be effectively used to treat male pattern hair loss in FtM patients by blocking the conversion of testosterone to dihydrotestosterone (DHT). However, finasteride may also slow or stop facial hair growth. Minoxidil (Rogaine) is an alternative topical agent that could be applied directly to the scalp.
Patients who do not achieve amenorrhea with testosterone alone may benefit from the addition of a progestin (i.e. injectable birth control, or levonorgestral-releasing intra-uterine device). GnRH analogues could also be used to suppress menses and the expression of endogenous female hormones. Danazol, a weaker androgen, is sometimes used to arrest menses and to achieve very mild virilization (100-200 mg twice daily). Endometrial ablation can also be considered.
Vaginal dryness and atrophy may result, especially post-oophorectomy. Topical estrogen can be used as in postmenopausal cis women. The little that is absorbed systemically is unlikely to interfere with masculinization if testosterone is maintained. Consider changing from a biweekly testosterone injection to a weekly injection at half the biweekly dose or decreasing the dose.
Some patients experience significant pelvic pain secondary to uterine cramping following orgasm. The mechanism of this pain is poorly understood but the use of non-steroidal anti-inflammatories approximately one hour prior to sexual activity may prevent this occurrence. Patients may also obtain some relief by adjusting the testosterone route or frequency to minimize serum peaks and troughs. Definitive management with hysterectomy may also be an option, especially if other motivating factors for this procedure are present.
The peaks and troughs of injectable testosterone may lead to fluctuations in energy and mood. Some patients may report significant fatigue and poor mood as serum levels fall leading up to the next injection. If this is a problem, increasing the frequency of injections or changing to a transdermal route of administration in order to attain a more steady serum level is often helpful.
Testosterone therapy tends to increase libido in FtM patients and hypersexual behaviour can occur. If this is distressing to the patient, a trial of a low dose SSRI may be considered.
Side effects of increased aggression or hostility are unusual, however, providers should monitor patients for any indication of behavioral changes.
If hormone therapy is discontinued, it is preferable to slowly taper the dose over a period of several weeks in order to minimize the side effects that may be associated with a more sudden change in serum hormone levels.
Elderly FtM Patients