Highlights
- Prior to beginning hormone therapy it is recommended that patients have an official diagnosis of gender dysphoria made by a mental health professional.
- The mainstay of cross-sex hormone therapy in MtF patients is estrogen (oral, topical, or injectable) and an anti-androgen.
- Absolute contraindications for hormone therapy must be ruled-out prior to initiation of treatment.
- Ever effort should be made to control medical conditions that may be exacerbated by estrogen prior to the initiation of estrogen.
- The physical changes caused by estrogen vary from patient to patient and range from irreversible to reversible. The total physical transition takes approximately two years.
- Routine follow-up is required with regular bloodwork and estrogen and anti-androgen dose adjustments as needed.
Overview
- Assessment
- Hormone Contraindications
- Hormone Risk Management
- Fertility
- Hormone Effects
- Hormone Regimens
- Patient Readiness
- Monitoring and Follow-up
Assessment
The assessment period is important for establishing a rapport with the patient, and establishing goals of treatment. The assessment period usually takes place over several office visits although the number of visits or time in between visits is less important than ensuring the following areas are covered.
Fast Tracking:
It may be appropriate to shorten the assessment period in the following circumstances:
- A patient who is already using illicit hormones;
- A patient who has marked distress regarding their gender presentation;
- A patient whose medical and/or gender history is well known to you prior to them seeking hormone therapy;
- If another knowledgeable provider has recently performed part or all of the assessment and the associated records are available for review or a conversation with the prior provider can be arranged.
Before beginning a discussion surrounding the details of hormone therapy it is important to have addressed the following areas:
- History and physical exam
- Mental health: Ensure all mental health concerns have been adequately addressed by yourself or another qualified mental health professional; an official diagnosis of gender dysphoria is also most commonly attained prior to beginning treatment and is a requirement prior to a specialist referral in Moncton.
- Screening and Preventative care: Ensure all aspects of the patient’s health have been reviewed and addressed as needed. It is especially important to minimize the risks of hormones by maximizing overall health (smoking cessation, regular exercise, weight loss, etc.). In addition, an ECG should be completed in patients over age 40 and a stress test should be considered if the patient has risk factors for coronary artery disease.
- Baseline bloodwork: CBC, liver transaminases, creatinine, urea, electrolytes, glucose, cholesterol, testosterone, estradiol, prolactin, luteinizing hormone, Hepatitis B, C screening.
- There is no consensus as to what gender should be listed on laboratory requisition. For a patient who has undergone neither medical nor surgical management, the natal sex is usually used. If a patient has undergone medical or surgical interventions, the use of the sex opposite to their natal sex is recommended (i.e., M for FtMs and F for MtFs). For patients who are currently transitioning or have partially transitioned, one may need to vary the reported sex depending on the lab test. For example, for MtF patients beginning feminizing therapy, male laboratory norms may be more appropriate for creatinine and cholesterol, but female values may be more relevant for testosterone levels.
Hormone Contraindications
After a history, physical, and bloodwork have been completed you should have all the information you need to determine if the patient has any contraindications to hormonal therapy.
Absolute contraindications to estrogen:
-
- Unstable ischemic cardiovascular disease
- History of an estrogen-sensitive neoplasm
- End-stage chronic liver disease
- Psychiatric conditions which limit the ability to provide informed consent
- Hypersensitivity to one of the components of the formulation
If estrogen is absolutely contraindicated, patients may still benefit from the use of anti-androgens.
Hormone Risk Management
A number of health risks have been thought to be related to feminizing therapy and some pre-exisiting medical conditions and risk factors may increase the risks associated with estrogen and anti-androgens. The level of risk depends on the medication itself (dose, route), and the patient’s clinical characteristics (co-morbidities, family history, health habits). It is important to discuss risks and benefits with the patient and take reasonable measures to reduce all risk factors associated with relevant conditions. While ideally it is best to delay hormone treatment until major risk factors are controlled, keep in mind that denying access to hormones can do much more harm than some of the possible risks of treatment. With informed consent and appropriate support many providers will initiate hormones in patients with higher risk profiles when the improved quality of life/chance of survival outweighs the potential risks of hormones.
Risks:
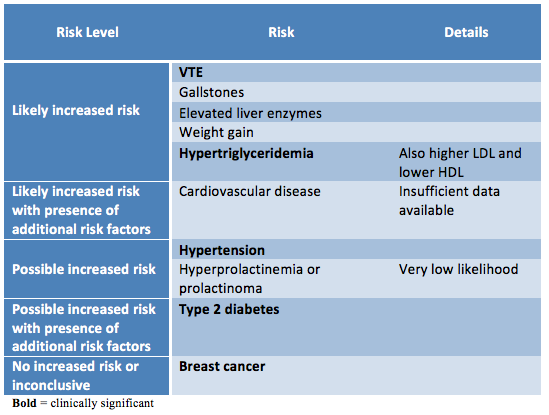 |
| Adapted from the WPATH Standards of Care version 7 |
Management:
- Stable ischemic heart disease: Consider cardiology consult, ensure optimal medical and/or surgical management as indicated, optimize risk factors. Consider transdermal and/or lower dose estrogen.
- Other cardiac disease: Consider cardiology referral.
- Cerebrovascular disease: Consider neurology referral, ensure optimal medical and/or surgical management as indicated, optimize risk factors. Consider transdermal and/or lower dose estrogen.
- Personal history of venous thromboembolism or hypercoagulable state: Minimize concomitant risk factors, consider prophylactic anticoagulation, consider hematology referral, consider prescribing regular Aspirin. Consider transdermal and/or lower dose estrogen.
- Family history of abnormal clotting: Consider hematology referral, rule out genetic clotting disorder, consider prophylactic anticoagulation. Use transdermal estrogen.
- Uncontrolled hypertension: Address barriers to control, use spironolactone as anti-androgen, add additional antihypertensives as needed (avoid ACEs/ARBs with spironolactone), consider a cardiac stress test. Encourage deferral of estrogen initiation until adequate control achieved, consider transdermal estrogen.
- Uncontrolled diabetes: Address barriers to control, refer to a dietician, encourage lifestyle changes, start antihyperglycemic agents as indicated, consider cardiac stress test. Encourage deferral of estrogen initiation until adequate control achieved, consider transdermal route.
- Marked hypertriglyceridemia: Address barriers to control, refer to a dietician, minimize alcohol consumption, consider antilipemic agents as indicated, consider endocrinology referral. Encourage deferral of estrogen initiation until adequate control achieved, consider transdermal estrogen.
- Smoking addiction: Encourage smoking cessation and offer medical assistance or negotiate a decrease in smoking, consider cardiac stress test (especially if additional cardiac risk factors present). Consider transdermal estrogen.
- Metabolic syndrome: Dietary and medical management of components as indicated, consider cardiac stress test. Encourage deferral until components adequately managed, consider transdermal estrogen.
- Severe refractory/focal migraines: Consider neurology referral, consider daily migraine prophylaxis, ensure other cerebrovascular risk factors are optimized. Consider transdermal estrogen.
- Seizure disorder: Consider neurology referral, consult with pharmacist re: interaction of estrogen and anti-convulsants.
- Hyperprolactinemia: Refer to endocrinology. Defer initiation of estrogen until etiology established then treat cause.
- History of benign intracranial hypertension: Consider referral to neurology/neurosurgery.
- Hepatic dysfunction: Address etiology as indicated (decreased alcohol consumption, weight loss if non-alcoholic fatty liver disease), consider GI/hepatology referral. Use transdermal or IM estrogen.
- Strong family history of breast cancer: Refer to genetics/familial breast cancer program for further risk stratification and BRCA 1/2 testing as indicated.
- Personal or family history of porphyria: Consider referral to a specialized clinic or internist with porphyria experience.
- Obesity: Encourage weight loss, consider referral to a dietician, encourage regular exercise.
Fertility
Another important risk to address is how hormone treatment may effect reproduction. Patients who never underwent puberty of their natal sex because they took hormone blockers (GnRH analogues) did not develop the secondary sex characteristics to produce the gametes of their natal sex. However, in patients who did undergo puberty of their natal sex, if that individual has not had complete gender-affirming surgery, it may be possible to stop hormones long enough for natal hormones to recover, allowing the production of mature gametes. It is unknown how long a patient would need to be off hormone therapy for this to potentially occur but extrapolated evidence suggests anywhere from 6 months to 3 years. Options should be discussed with patients prior to initiating hormone therapy. Patients should be informed about sperm preservation and encouraged to consider sperm banking. While there is availability of these services in Moncton (despite the wording of the website), unfortunately the cost is not covered.
Birth Control: Hormone impact on fertility in MtF patients is variable and barrier methods of contraception should be discussed depending on sexual practices.
Hormone Effects
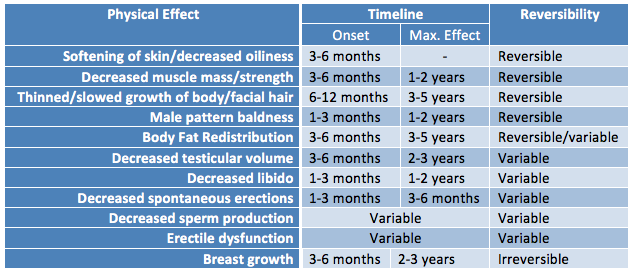
Hormone therapy will lead to many physical changes on a variable timeline that lasts on average two years. Physiologic responses to hormone therapy are quite variable and it is impossible to predict exactly what changes each patient can expect. The underlying body structure will not change with hormones. Trans women will maintain narrow hips and existing facial bone structure and the pitch of the voice will not be affected. Cross-sex hormones also do not affect adult height. Changes that do occur include: softened skin with decreased oiliness, decreased muscle mass and strength, thinned or slowed growth of body and facial hair, male pattern baldness, body fat redistribution, decrease testicular volume, decreased libido, decreased spontaneous erections and erectile dysfunction, decreased sperm production, and breast growth. Changes range from reversible to irreversible. It is important that patients have realistic expectations regarding the possible changes with hormones.
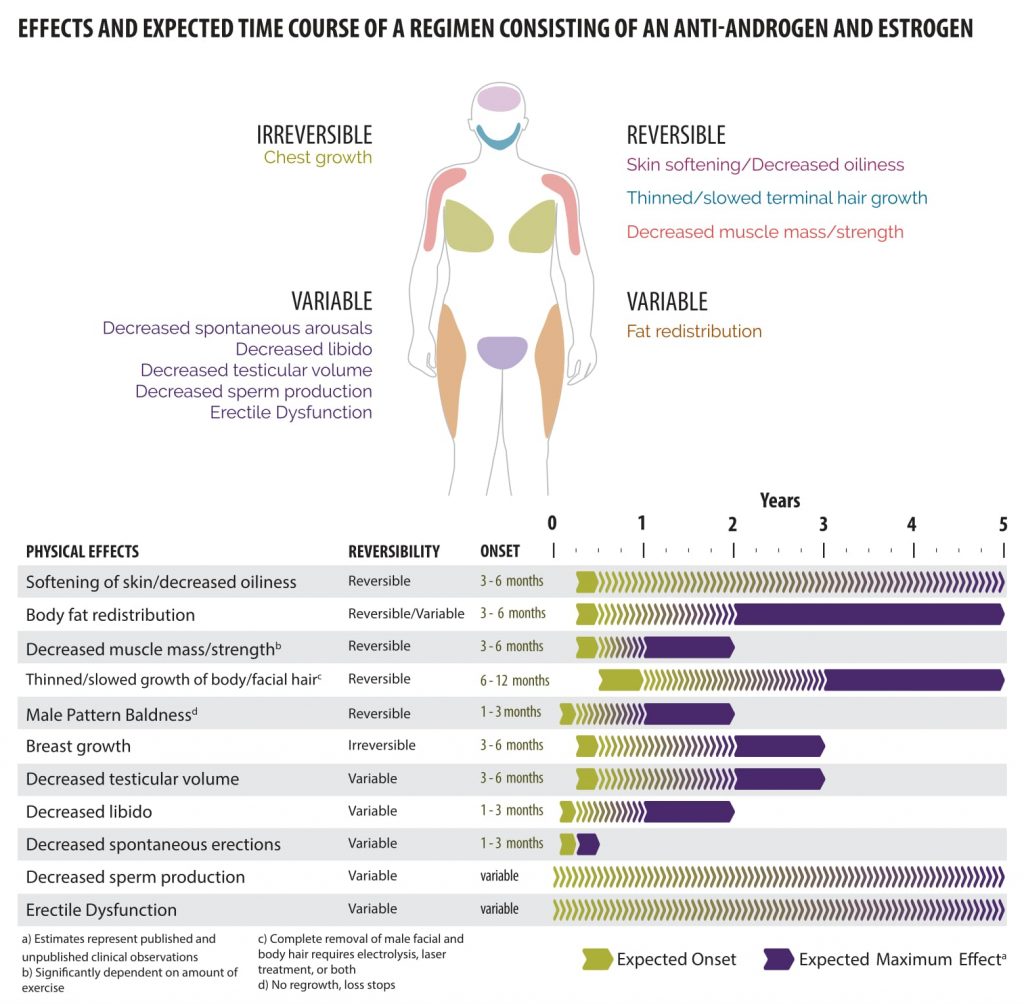 |
| Courtesy of Rainbow Health Ontario |
Sexuality
Exogenous hormones may have an impact on sexual attraction. Patients may notice an expansion of their sexual interests or a shift in their sexual orientation, which can be transient or permanent This is often unforeseen and may create challenges for existing relationships. This effect has been most commonly noted with masculinizing therapies, but should be discussed as a possibility in all patients considering hormone therapy. Integrating an ongoing sexual health history is helpful in assessing for changes in risks for sexually transmitted infections and unplanned pregnancy.
Hormone Regimens
No controlled clinical trials have been conducted to assess the safety or efficacy of feminizing/masculinizing hormones in producing physical transition. A wide variety in dosing and types of hormones exists. Selecting the appropriate hormonal therapy will depend on patient preference, patient goals of therapy, pre-existing medical conditions, and cost. Expected rate and degree of physical change will depend on dose and route of administration, age, genetics, body habitus, and lifestyle.
Medications used in MtF transition include both estrogens and anti-androgens (spironolactone, cyproterone), in order to achieve adequate androgen suppression. The use of progestins in trans women remains controversial. There has not been a clear feminizing benefit shown with the use of progestins, although some clinicians feel it has been a useful adjunctive medication (see below).
In terms of oral estrogens, estradiol (Estrace) is more commonly prescribed than the conjugated estrogen (Premarin) because of its preferable safety profile. Transdermal and parenteral estrogens appear safer from a thrombogenic perspective compared to oral estrogens as they avoid the first pass effect and also have fewer hepatic side effects. Transdermal or parenteral estrogens are recommended for patients who are over the age of 40 or have risk factors for cardiovascular or thromboembolic disease. Some patients report faster breast development with intramuscular estrogen. Ethinyl estradiol is associated with an increased risk of venous thromboembolism and is therefore contraindicated in transgender management. Use of anti-androgens without estrogen can result in decreased bone density. Finasteride may be added to an estrogen and anti-androgen regime to reduce androgenic hair loss.
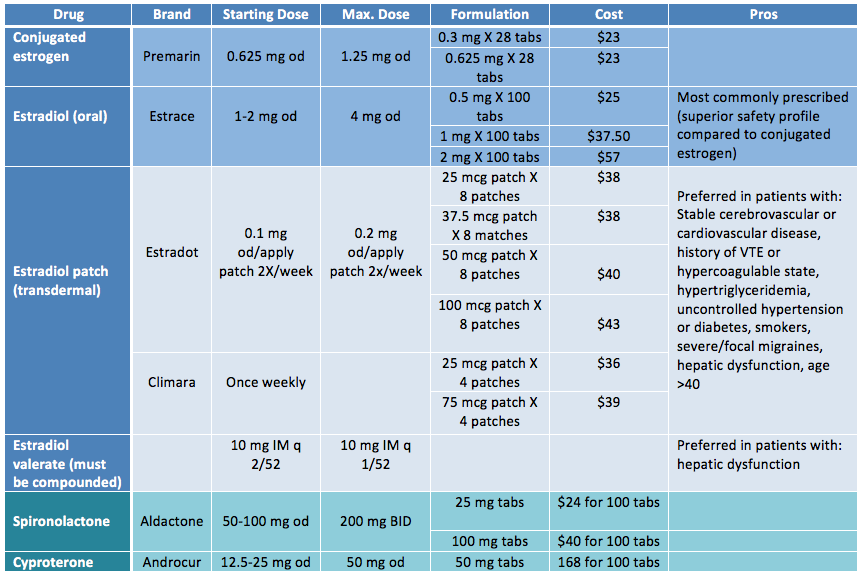 |
| Adapted from Rainbow Health Ontario |
Patient Readiness
Ensure that the patient has realistic expectations about what type of changes are possible as well as the timeline they can expect the changes to occur. Ensure the patient has a thorough understanding of the possible risks and complications of hormone therapy and that they understand that in some circumstances, they may develop a medical condition in the future the precludes then from continuing hormone therapy (e.g., certain types of cancer). It is also important to discuss fertility with all patients prior to starting hormonal therapy.
Gender transition and hormonal therapy represent significant change in a patient’s life and psychosocial support is important. Although not required for hormonal therapy, many patients may benefit from a trans-positive therapist.
Patients may choose to undergo a social transition either before, after, or in parallel with cross-sex hormone therapy. Social transitioning is a period of time in which transgender individuals live full-time in their affirmed gender. The purpose of this is to help patients confirm their affirmed gender and evaluate their ability to function as a member of that gender. It also provides insight into the adequacy of their social, economic, and psychological support. During social transitioning, the person’s feelings about the social transformation (including coping with the reaction of others) is a major focus of the counselling.
Additionally, patients should be encouraged to discuss their transition with family and friends as they too will be impacted by the patient’s transition. Consider asking the following questions:
- Who makes up your support system? How readily accessible are they?
- Do you know anyone else who has transitioned? What were the major struggles they had?
- What are the challenges you foresee with your family/friends?
- How will you manage your transition at work/school?
- How can you address those issues?
Attaining Consent
A patient must demonstrate an understanding of the risks and benefits of hormone therapy as well as existing alternatives. They must understand that physical transition is variable and no guarantees about final appearance can be made. Consent forms are not used by all physicians in Moncton but they are recommended by the WPATH. A sample consent from for female hormones/anti-androgens is available here, adapted from the template by the Sherbourne Health Centre.
Monitoring and Follow-up
See Appendix D and E in the Guidelines and Protocols for Hormone Therapy and Primary Health Care for Trans Clients for a preventative care checklist for trans women.
Patient’s should be followed up at 1, 3, 6, and 12 months during the first year and then every 6-12 months thereafter, or more frequently if needed. Each follow-up should consist of a functional inquiry, targeted physical exam, preventative care/counselling, and bloodwork. The best assessment of efficacy of the hormone therapy is the clinical response – the patient’s physical changes. However, in order to more rapidly predict the appropriate hormone dosages the hormone levels are often used as guidance.
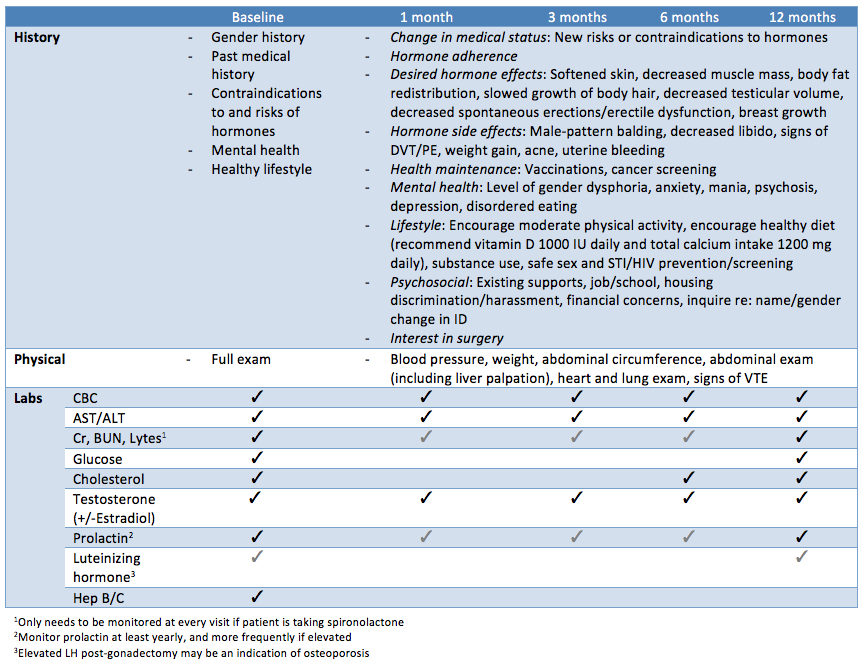 |
| Adapted from Rainbow Health Ontario |
The starting dose of estrogen can be maintained for 1-2 months then a dose increase can be considered if tolerated. A sample dose titration would be: 2 mg estrace + 50 mg spironolactone x 4-6 weeks, then 3 mg estrace + 100 mg spironolactone x 4-6 weeks, then 4 mg estrace + 150 mg spironolactone x 4-6 weeks. Both serum estradiol and serum testosterone should be monitored. However testosterone levels may be the more useful test for monitoring in MtF patients given the cyclical variation of estradiol in cis women. Testosterone levels should be suppressed below the upper limit of the normal female range (<1.70 nmol/L) and estradiol levels should be within the range of a premenopausal cis woman but well below supraphysiologic levels. However, the patient may have clinically relevant results without total suppression of testosterone because of the androgen blockade, which is not easily measured, and the clinical results are more important than the hormone levels. Most patients attain considerable feminization at estradiol levels between 200-500 pmol/L. According to the American Endocrine Society Guidelines, serum estradiol levels should not exceed the mean daily level for cis women (approximately 700 pmol/L). Supraphysiologic estrogen may lead to increased risk for thromboembolic disease, liver dysfunction, and hypertension.
Note: Bloodwork can monitor serum estradiol levels but not the levels of conjugated or synthetic estrogens (i.e. Premarin). This is another reason why synthetic or conjugated estrogens are undesirable, as they lead to more difficult monitoring, thus increasing the risk of administering supraphysiologic doses and increasing the risk of venous thromboembolism.
Clinicians should measure prolactin levels at baseline and then at least annually during the transition period and every 2 years thereafter. Because the major presenting findings of microprolactinomas (hypogonadism and sometimes gynecomastia) are not apparent in trans women, clinicians may perform radiologic examinations of the pituitary in those patients whose prolactin levels persistently increase despite stable or reduced estrogen levels. Some transgender individuals receive psychotropic medications that can increase prolactin levels.
Once patients have achieved maximal feminizing benefit from hormones (typically two or more years) they remain on a maintenance dose. Maintaining body changes generally requires lower hormone doses compared to initial induction. The maintenance dose is then adjusted for change in health conditions, aging, or other considerations (e.g., lifestyle changes).
In patients concerned about limited erectile function, consider adjusting the dose of hormones first, balancing the degree of feminization with the level of erectile function. If unsuccessful, consider erection-enhancing drugs (e.g. phosphodiesterase type 5 inhibitors like Sildenafil). An increased risk of prostatitis has been noted in MtF patients in the first few years of transition, hypothetically linked to decreased ejaculation and stagnate prostate secretions.
Patients who experience a low libido may need to have their testosterone maintained at a higher level. Testosterone dosing is similar to treating sexual dysfunction in ciswomen- 0.2–1 mg of topical testosterone daily. Careful monitoring is required for signs of masculinzation and regular bloodwork should be done initially until a therapeutic dose is established. A trial of progestin can also be tried. The common doses of progestins are micronized progesterone 100-400 mg daily or medroxyprogesterone acetate 5 – 30 mg daily. Some clinicians advise limiting progestin treatment duration to a maximum of 2-3 years. Some clinicians require patients to sign an extra consent form if progesterone treatment is started.
Patients may occasionally need to reduce or temporarily stop their hormone therapy prior to upcoming procedures such as surgery or sperm banking. MtF patients should discontinue their estrogen 2-4 weeks prior to any major surgery to reduce the risk of thromboembolic events.
Androgen blockers can normally be tapered post-orchiectomy over a 4-6 week course. Ongoing estrogen supplementation is still required to preserve bone strength. Younger clients may require the same dose of estrogen post-operatively as they did pre-operatively, however older clients may be able to be maintained on a lower (i.e. starting) dose.
In patient greater than 50 years of age who have been on estrogen for several years, the dose may be reduced to the dose typically given to post-menopausal cis women (i.e. 50-100 mcg estrogen twice per week). For trans women starting transition over 50 years old, an ‘active period’ of treatment with suggested doses used for younger patients may be considered following a thorough assessment and discussion of relative risks and benefits.
If hormone therapy is discontinued, it is preferable to slowly taper the dose over a period of several weeks in order to minimize the side effects that may be associated with a more sudden change in serum hormone levels.
Having undetectable levels of testosterone or estradiol in elderly patients is not abnormal and should not be an indication to adjust therapy.